13+ match each diagram to the atom or ion it represents
Displacement vectors P PD corresponding to local offsets between the A- and B-site sublattices were calculated by first determining atomic positions by fitting each atom site in the image to. When it reverts to normal state it releases 048 gigjoules per kilogram.
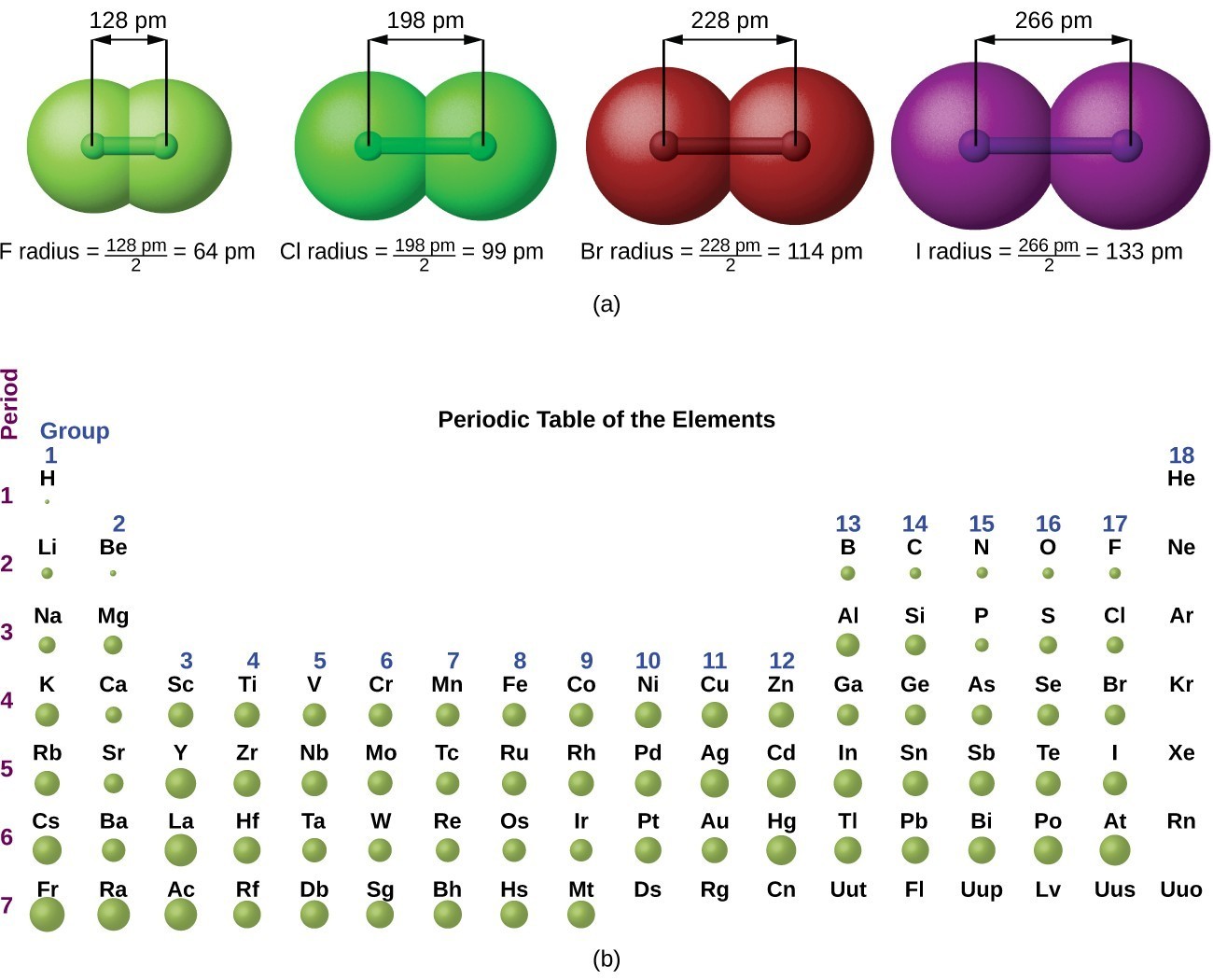
Periodic Variations In Element Properties Chemistry For Majors
A b 0 c 0 d 0 e f - g 0.

. A single electron can make all the difference in the properties of an atom. Select every molecule or ion that activates reaction 3. C TEM image of Cu nanocubes.
Carbon dioxideA gas mixture such as air contains a variety of pure gases. For example electron ionization EI gives a high degree of fragmentation yielding highly detailed. The electron is one of the most important factors in determining how an atom will react with another atom or molecule.
The oxygen evolution reaction OER is a key process in such conversions having. Three principal eigenvectors. Its density is about 70 higher than that of lead and slightly lower than.
Insolubility is the opposite property the inability of the solute to form such a solution. Part B Rank the following compounds in order of decreasing. Gas is one of the four fundamental states of matter the others being solid liquid and plasma.
This can easily be observed in a. Two electrons in a helium atom are aligned in a metastable state one electron each in the 1s and 2s atomic orbitals with both electrons having parallel spins the so-called triplet spin state if you want the details. A n 3 l 1 ml 1 ms -12.
Hydrogen fluoride HF carbon tetrachloride CCl4 and dichloromethane CH2Cl2. ASCII characters only characters found on a standard US keyboard. Give the set of four quantum numbers that could represent the electron lost to form the K ION from the K atom.
Oxygen or compound molecules made from a variety of atoms eg. Must contain at least 4 different symbols. 69-613 odd Second language pg 104 ch 6.
The overlaid schematic represents a possible 2D structural model of Ti 1 rGO the rGO structure is represented in white around a Ti atom represented with a green ball determined from EELS. Draw the most stable and the least stable conformations for each of the following compounds. A pure gas may be made up of individual atoms eg.
It is a metal that belongs to the first transition series and group 8 of the periodic tableIt is by mass the most common element on Earth right in front of oxygen 321 and 301 respectively forming much of Earths outer and inner coreIt is the fourth most common. The design and synthesis of efficient electrocatalysts are important for electrochemical conversion technologies. Quantum mechanics is the study of matter and its interactions with energy on the scale of atomic and subatomic particlesBy contrast classical physics explains matter and energy only on a scale familiar to human experience including the behavior of astronomical bodies such as the moon.
Study with Quizlet and memorize flashcards containing terms like 1. Pt nanoparticles with about 3070 atoms show the highest positive charge on each Pt atom which indicates the strongest electronic metalsupport interaction. Please contact Savvas Learning Company for product support.
Uranium has the highest atomic weight of the primordially occurring elements. Water is a tasteless odorless liquid at ambient temperature and pressureLiquid water has weak absorption bands at wavelengths of around 750 nm which cause it to appear to have a blue colour. Ionization occurs in the ion sourceThere are several ion sources available.
It is a silvery-grey metal in the actinide series of the periodic tableA uranium atom has 92 protons and 92 electrons of which 6 are valence electrons. Making the stuff is easy. In mass spectrometry ionization refers to the production of gas phase ions suitable for resolution in the mass analyser or mass filter.
6 to 30 characters long. Water is an oxygen hydride consisting of an oxygen atom that is covalently bonded to two hydrogen atoms It has a role as an amphiprotic solvent a member of greenhouse gas a human metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite and a mouse metabolite. Schematic in a represents a biological ion channel with an angstrom-sized asymmetric ion selectivity filter and a nanometer-sized cavity for fast ion transport Spheres.
The extent of the solubility of a substance in a specific solvent is generally measured as the concentration of the solute in a saturated solution one in which no more solute can. They are so small that accurately predicting their behavior using classical physics as if they were tennis balls for example is not possible due to quantum effects. Classical physics is still used in much of modern science and technology.
These nanotubes were grown on silicon substrates using an improved chemical vapor deposition CVD method and represent electrically uniform arrays of single-walled carbon nanotubes. The skeleton of the bicarbonate ion HCO3- is shown here. The first step in the catabolism of most amino acids is the removal of the nitrogen atom by transfer to an alpha-keto acid a reaction catalyzed by an enzyme called a transaminase.
Sapling HW Ch 17. Orange oxygen blue. A The schematic diagram of the setup of operando time-resolved XAS experiments.
A noble gas like neon elemental molecules made from one type of atom eg. B The cartoon representation of customized operando XAS cell. Uranium is a chemical element with the symbol U and atomic number 92.
Iron ˈ aɪ ər n is a chemical element with symbol Fe from Latin. D TEM image of CuO x. Each has advantages and disadvantages for particular applications.
Match each patients condition with the reasons for the observed symptoms. It is an oxygen hydride a mononuclear parent hydride and an inorganic hydroxy. The shortest carbon nanotube can be considered to be the organic.
The symmetric Pd 13 O 4 showed higher activation energy for CO oxidation via reaction of an adsorbed CO molecule with one of the oxygen atoms of the Pd 13 O 4 cluster. One molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom. Where X represents the central atom in each of the following compounds or ions.
The dataset was then arranged as N D where N represents background-subtracted spectra at different points and D the pixel intensity of each individual signal. Water is the chemical substance with chemical formula H 2 O. Drag the appropriate items to their respective bins.
What is the predominant intermolecular force in the liquid state of each of these compounds. In chemistry solubility is the ability of a substance the solute to form a solution with another substance the solvent. Every solid liquid gas and plasma is composed of neutral or ionized atoms.
An atom is the smallest unit of ordinary matter that forms a chemical element. Atoms are extremely small typically around 100 picometers across. Ferrum and atomic number 26.
The observation of the longest carbon nanotubes grown so far around 05 metre 550 mm long was reported in 2013. Determine the formal charge on the nitrogen atom in each of the following structures.

A An H 2 O Group Held In A Structure Solely By Hydrogen Bonds The Download Scientific Diagram

High Energy Density Physics With Intense Ion And Laser Beams Gsi

Oneclass Match Each Diagram To The Atom Or Ion It Represents

Guiding Of Charged Particles Through Capillaries In Insulating Materials Sciencedirect

Pdf Resonant Photo Ionization Of Yb To Yb2

Electron Capture Dissociation And Drift Tube Ion Mobility Mass Spectrometry Coupled With Site Directed Mutations Provide Insights Into The Conformatio Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 C4cp05136j

Pdf Inorganic Chemistry By Gary L Miessler Maitha Al Rumaithi Academia Edu

The Most Precise Atomic Mass Measurements In Penning Traps Sciencedirect
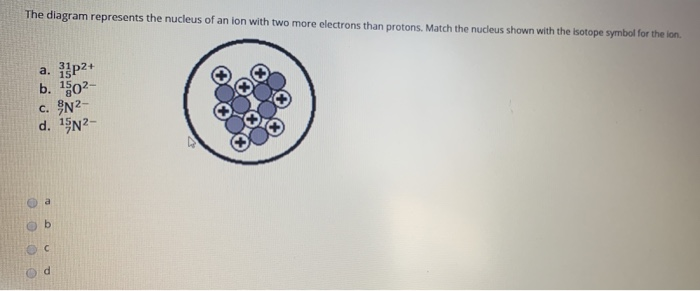
Solved The Diagram Represents The Nucleus Of An Ion With Two Chegg Com
Laser Cooling Of Antihydrogen Atoms Nature

Pdf Chapter 1 Chemistry The Study Of Change Problem Categories Hugo David Academia Edu

Part A Match Each Diagram To The Atom Or Ion It Repres Itprospt

Characterization Of Laser Generated Aluminum Plasma Using Ion Time Of Flight And Optical Emission Spectroscopy Journal Of Applied Physics Vol 122 No 20

Atoms Isotopes Ions And Molecules The Building Blocks Openstax Biology 2e
Imaging Plasma Density Structures In The Soft X Rays Generated By Solar Wind Charge Exchange With Neutrals Springerlink

Roadmap On Photonic Electronic And Atomic Collision Physics Iii Heavy Particles With Zero To Relativistic Speeds Iopscience

Pdf Chapter 1 Chemistry The Study Of Change Problem Categories Daniel Garcia Academia Edu